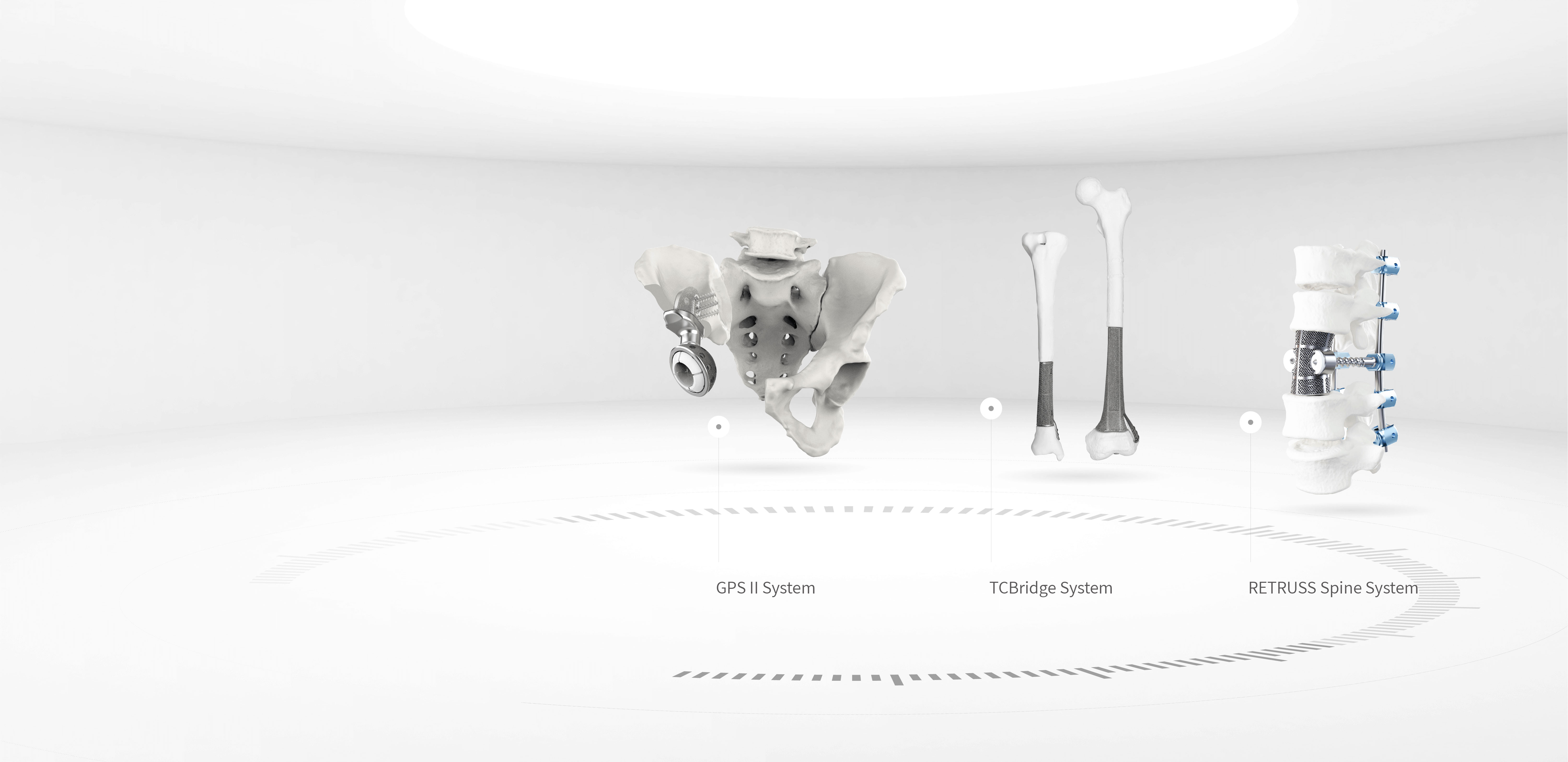
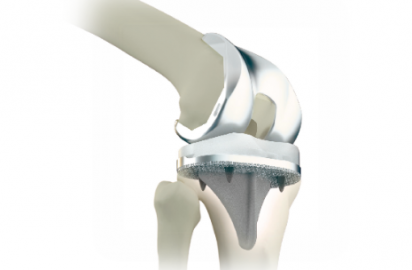
Metal Additive Manufacturing Technology
3D Accurate Construction Technology (3D ACT)AK Medical is one of the earliest companies in the global orthopedic field to conduct research on the application of metal additive manufacturing technology. We are at the forefront of international advancements. By applying metal additive manufacturing technology and clinical imaging data, we can precisely construct the bone-like trabecular structure (3D ACT). It provides a three-dimensional penetrating space for microvascular and neo-bone growth and achieves osseointegration at the prosthesis-bone interface. It also prevents prosthesis loosening and prolongs its service life.
Vacuum Plasma Spraying Technology
Supravit®-enabled HA CoatingVacuum plasma spraying technology (VPS) has been well adopted and used by JRI, a wholly owned subsidiary of AK Medical, for 33 years. A wide range of clinical applications have shown that HA coating, with its proven safety and effectiveness enabled by the patented Supravit® technology, increases prostheses’ post-surgery immediate stability and long-term osseointegration.
Smart Orthopedic Technology
Visual Treatment SolutionThe AK Medical intelligent orthopedic equipment technology covers such equipment as orthopedic diagnosis and treatment software and intelligent surgical robots. Through 3D surgical intelligent planning software, the most suitable surgical plan can be formulated before surgery; the whole surgical process is visualized and tracked in real time to assist in the accurate implantation of the prosthesis; in this way, the accuracy and safety of the surgery can be significantly improved.
Orthopedic Total Customization Technology
an orthopedist-engineer co-workplatform empowered by AK engineering capabilitiesAK Medical is one of the world’s first providers of personalized orthopedic diagnosis and treatment technology services. We are a pioneer of ICOS (Innovative Custom Orthopedic Solution) technology. We offer personalized surgical planning programs that satisfy the individualized demands of patients and provide personalized orthopedic implant product systems and complete instrument sets for clinical treatment to enhance treatment outcomes.